Archive
Cytophage Reports Successful Results and Data from Second EU Pilot Study for AviPhage
 | |||||||||
 |  |  | |||||||
WINNIPEG, September 23, 2025 — TheNewswire - Cytophage Technologies Ltd. (“Cytophage” or the “Company”) (TSXV: CYTO, FSE: 70G) today announced positive results from its follow-up European pilot study of AviPhage, the Company’s proprietary poultry-based bacteriophage (phage) product for broiler chickens. These results reinforce the efficacy of AviPhage as a strong contender for antibiotic replacement in poultry farms, provide valuable new data that builds upon the initial pilot study findings, and mark another milestone in advancing the product toward regulatory approval and commercial launch.
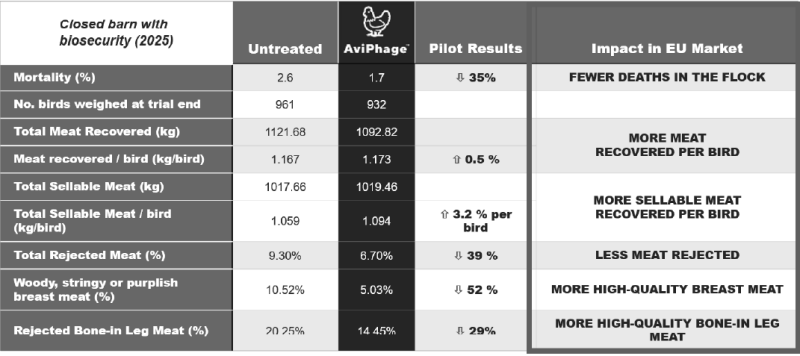
The purpose of this follow-up study was to evaluate the efficacy of AviPhage on European poultry within a working-barn setting. AviPhage-treated birds showed lower mortality and produced higher quality meat per bird compared to the control group birds. Overall, AviPhage’s broiler chickens matched or outperformed control group chickens on several key performance indicators, resulting in substantial improvement in the quality and quantity of the sellable meat which can have a direct economic impact for poultry producers.
“The results from this study underscore the real-world impact AviPhage can deliver – reducing reliance on antibiotics without sacrificing high quality food production. With this new data, we are well-positioned to accelerate regulatory engagement and commercial partnerships as we move closer to market introduction in Europe,” said Dr. Steven Theriault, CEO of Cytophage.
Pilot Study Results - Highlights
-
DECREASED MORTALITY: AviPhage treatment reduced mortality by 35%, outperforming the control group and maintaining a mortality rate well below the European Food Safety Authority (EFSA) standard for this breed. AviPhage-treated birds remained healthy throughout the trial period.
-
INCREASED SELLABLE MEAT: Each AviPhage-treated bird yielded 3.2% more sellable, high-quality meat compared to controls.
-
DECREASED REJECTED MEAT: Rejected meat rates dropped by 39% in the AviPhage group.
-
INCREASED HIGH QUALITY BREAST MEAT: Incidence of poor breast meat (woody, stringy) dropped by 52%.
-
INCREASED HIGH QUALITY BONE-IN LEG MEAT: Rejection rates for bone-in leg meat decreased by 29%.
-
INCREASED MEAT RECOVERED PER BIRD: AviPhage treatment saw an average increase of 0.5% meat recovered per bird.
In addition to Cytophage’s first EU pilot study earlier this summer where effective E. coli containment and decreased mortality were observed, this study’s findings further validate AviPhage’s commercial potential as a next-generation solution for poultry producers seeking to reduce antibiotic use while maintaining or enhancing productivity.
Next Steps
The results of this study advance Cytophage’s goal to secure regulatory approval and product registration across the EU. The studies have demonstrated product efficacy and the capacity of the AviPhage to replace antibiotics within the working barn setting. More studies will be required for the broader EU regulatory application. At the same time, the Company is continuing its efforts to further validate AviPhage’s effectiveness in the global market.
About Cytophage
Cytophage Technologies (TSXV:CYTO / FSE: 70G) is a pioneering biotechnology company dedicated to bacteriophage research, product development and commercialization. Bacteriophages are viruses that only infect and kill bacteria. These natural killers of bacteria can overcome cellular or organism-level defences. They are nature’s version of antibiotics.
Cytophage is improving bacteria’s natural predators with environmental as well as genetic modifications to bring safe and toxin-free killer solutions to large addressable markets with an initial focus on animal health which offers a fast-track to near-term revenue. As a leading bacteriophage manufacturer in Canada and powered by a large library of strains, Cytophage is committed to addressing the global challenge of antibiotic resistance (AMR).
The WHO predicts that AMR will be the leading cause of human mortality by 2050. Many countries have already banned or limited preventative antibiotic use in animal production including 27 EU countries, US, Canada, Brazil, Bangladesh, India and Mexico. In addition to that, consumers all over the world are demanding organic and antibiotic-free products.
Cytophage is using a de-risked and patented technology to advance innovative and cost competitive products that harness the power of bacteriophages to combat bacterial infections affecting human health, animal health, and food security.
For more information on Cytophage Technologies and its innovative work in bacteriophage therapy, please visit www.cytophage.com.
For further information please contact:
Heather Medwick, Chief Operating Officer
heather@cytophage.com
Cytophage Investor Alerts: https://cytophage.com/subscribe
Cautionary Statement on Forward-Looking Information
This news release contains “forward-looking information” and “forward-looking statements” (collectively, “forward-looking statements”) within the meaning of the applicable Canadian securities legislation. All statements, other than statements of historical fact, are forward-looking statements and are based on expectations, estimates and projections as at the date of this news release. Any statement that involves discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, assumptions, future events or performance (often but not always using phrases such as “expects”, or “does not expect”, “is expected”, “anticipates” or “does not anticipate”, “plans”, “budget”, “scheduled”, “forecasts”, “estimates”, “believes” or “intends” or variations of such words and phrases or stating that certain actions, events or results “may” or “could”, “would”, “might” or “will” be taken to occur or be achieved) are not statements of historical fact and may be forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of Cytophage to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Factors that could cause actual results to differ materially from those anticipated in these forward-looking statements are described under the caption “Risk Factors” in Cytophage’s Filing Statement dated January 30, 2024, which is available for view on SEDAR+ at www.sedarplus.ca. These risks include but are not limited to, the risks associated with the bacteriophage industry, such as operational risks in development or capital expenditures, the uncertainty of extensive regulatory approval requirements, government regulations, protection of intellectual property, product liability and rapid technological advancements. Forward-looking statements contained herein are made as of the date of this press release, and Cytophage disclaims, other than as required by law, any obligation to update any forward-looking statements whether as a result of new information, results, future events, circumstances, or if management’s estimates or opinions should change, or otherwise. There can be no assurance that forward-looking statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, the reader is cautioned not to place undue reliance on forward-looking statements.
Neither the TSXV nor its Regulation Services Provider (as that term is defined in the policies of the TSXV) accepts responsibility for the adequacy or accuracy of this news release.