Archive
Voyageur Pharmaceuticals Initiates First Commercial Shipments of Barium Contrast Products to Canadian Clinics
 | |||||||||
 |  |  | |||||||
Calgary, Alberta, Canada – August 21, 2025 – TheNewswire - Voyageur Pharmaceuticals Ltd. (TSX.V:VM) (OTC Pink:VYYRF) ("Voyageur" or the "Company"), a radiology contrast media company focused on vertical integration and supply chain security, today announces the first commercial delivery of the full suite of Voyageur’s Health Canada–approved barium contrast products. Further to the Company's news release dated June 4, 2025, the delivery was to a leading Canadian radiology provider who signed a purchase order in June 2025. Voyageur's product line is currently being evaluated by other private clinics, group purchase organizations and Provincial Health Care procurement groups.
The order follows successful product evaluations and regulatory milestones, with Voyageur hitting its milestone to deliver product by the end of August.
“This sale has many important aspects for the development of the Frances Creek project and anticipated global expansion,” said Brent Willis, President and CEO. “The data accumulated from the use of our products will be a critical component of our FDA filings to enter the US market and is expected to provide the economics to complete our final feasibility study to advance the Frances Creek project towards production.“
Approved Product Line Now Commercialized
Voyageur’s approved barium contrast agents are optimized for a range of gastrointestinal imaging applications:
-
SmoothX (2%w/v): For CT imaging of the GI tract; noted for its improved taste and tolerability.
-
SmoothHD® 105%: High-density suspension for upper GI and double contrast studies; delivering sharp mucosal detail.
-
SmoothLD® (60% w/v): Low-density suspension for single contrast GI studies.
-
VisionHD® (98% w/w): Powdered high-density suspension for upper GI double-contrast imaging.
-
VisionLD® (96% w/w): Powdered low-density suspension for single contrast studies.
Poised for Scalable Growth with Strategic Resource Advantage
With commercial sales now underway, Voyageur is focused on expanding distribution across Canada. This infrastructure is designed to scale alongside the Company’s Frances Creek Project in British Columbia, a 100% owned, pharmaceutical grade barium sulfate resource.
Management believes the key advantages of Frances Creek include:
-
Low-Cost Production– Estimated at ~C$620-$650/tonne, significantly undercutting foreign BaSO API imports that currently cost C$10,000/tonne.
-
Long-Term Supply– 121,000-tonne indicated and inferred resource valued at NPV C$344M
The resources are shown in the table below:
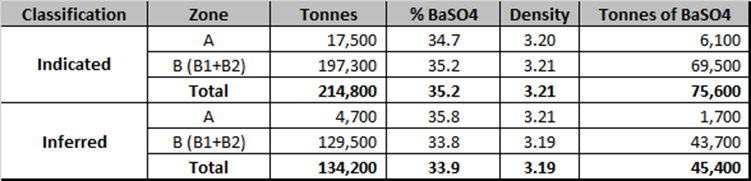
-
(1)Effective date for resources is January 11, 2022.
(2) The independent QP for this resources estimate is Yann Camus, P.Eng., SGS Geological Services.
(3) The base case is reported at a cut-off grade of 10% BaSO4.
(4) Open pit mineral resources are reported at a base case cut-off grade of 10% BaSO4 within a conceptual pit shell. Cut-off grades are based on a barite price, barite mining and processing recovery and mining and processing cost.
(5) The resources are presented in-situ and without dilution.
(6) Block fraction was applied to the mineral resources.
(7) Mineral resources that are not mineral reserves do not have demonstrated economic viability. The quantity and grade of reported Inferred Resources in this Mineral Resource Estimate are uncertain in nature and there has been insufficient exploration to define these Inferred Resources as Indicated or Measured. However, based on the current knowledge of the deposits, it is reasonably expected that the majority of Inferred Mineral Resources could be upgraded to Indicated Mineral Resources with continued exploration.
(8) This Resource Estimate has been prepared in accordance with CIM definition (2014).
(9) The density used for each block is based on grade and the following formula:
2.828+(0.0000009404*(BaSO4)3) - (0.000030963*(BaSO4)2) + (0.010623107* BaSO4)
(10) A variable BaSO4 capping grade was used by removing 10% of the grade on assay with lengths >5 m and resulting in an overall reduction of 5% of the barite content.
(11) Total may not add due to rounding.
-
Strategic Positioning– Enables North American supply chain independence and cost leadership.
-
Sustainability Focus– Plans for carbon-neutral operations utilizing advanced capture technology.
Barium contrast media play an essential role in gastrointestinal imaging, offering a reliable, radiopaque medium with favorable safety and cost profile compared to oral iodinated agents. Voyageur’s innovative formulations are designed to meet the needs of both adult and pediatric patients in a range of imaging contexts.
The Company believes this vertical integration strategy will enhance margins, ensure quality control, and create a lasting competitive edge in the barium contrast media market.
All inquiries for purchasing Voyageur's product line, please contact Ethan Mohan at ethan@vpharma.ca.
This news release has been reviewed and approved by Brad Willis, P. Eng., a “Qualified Person” under National Instrument 43-101, and the Chief Operating Officer and a director of the Company.
About Voyageur Pharmaceuticals Ltd.
Voyageur, a Canadian public company trading under the symbol VM on the Exchange, is in development of barium and iodine Active Pharmaceutical Ingredients (API) that offer high-performance and cost-effective imaging contrast agents. With a strategic focus on vertically integrating the barium and iodine contrast markets, Voyageur aims to become a key player by producing its own barium and iodine. In addition, Voyageur is pursuing the development of new endo fullerene drugs.
Voyageur's business plan is set to generate cash flow by partnering with established third-party GMP pharmaceutical manufacturers in Canada thereby ensuring the validation of its products by regulatory agencies worldwide. As Voyageur solidifies its presence in the market, it plans to transition into a high-margin domestic manufacturer of radiology drugs.
At the core of its operations, Voyageur owns a 100% interest in the Frances Creek Project. Currently, the world's pharmaceutical barium sulphate is almost entirely synthetically produced resulting in a less effective imaging quality product. Voyageur’s Frances Creek resource boasts a rare and exceptional grade mineral suitable for the pharmaceutical marketplace that Voyageur believes will replace the current synthetic products with higher quality imaging products.
Voyageur's ambitious vision is to become the first vertically integrated company in the radiology contrast media drug market. By controlling all primary input costs, from the sourcing of raw materials to final production, Voyageur believes it can ensure quality and cost efficiency. With its approach, it embodies the motto of "From the Earth to the Bottle," highlighting Voyageur's commitment to responsible sourcing and manufacturing practices.
For Further Information:
|
Brent Willis, CEO, |
Albert Deslauriers, CFO, |
|
Brent@vpharma.ca, 403-923-5944 |
Albert@vpharma.ca |
|
info@vpharma.ca |
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this news release.
Cautionary Statement Regarding “Forward-Looking” Information
This news release may contain certain forward-looking information and statements, including without limitation, statements pertaining to: the first deliveries of products beginning in August; the Company's expectation that the Frances Creek Project has enough supply to support 50 years of production; the Company's plans for carbon-neutral operations utilizing advanced capture technology; the Company's belief that its vertical integration strategy will enhance margins, ensure quality control, and create a lasting competitive edge in the barium contrast media market; the Company's aim to become a key player in the barium and iodine contrast markets; the Company's plan to transition into a high-margin domestic manufacturer of radiology drugs; the Company's belief that the Frances Creek Project's mineral will replace the current synthetic products in the pharmaceutical marketplace with higher quality imaging products; and the Company's belief that it can ensure quality and cost efficiency by controlling all primary input costs. All statements included herein, other than statements of historical fact, are forward-looking information and such information involves various risks and uncertainties. There can be no assurance that such information will prove to be accurate, and actual results and future events could differ materially from those anticipated in such information. A description of assumptions used to develop such forward-looking information and a description of risk factors that may cause actual results to differ materially from forward-looking information can be found in the Company's disclosure documents on the SEDAR+ website at www.sedarplus.ca. Voyageur does not undertake to update any forward-looking information except in accordance with applicable securities laws.